Outdated Content – please see most recent alerts.
Whatcom County Health Department Communicable Disease & Epidemiology Division
Who to Test for COVID-19:
COVID-19 testing is allowed based on clinician’s judgement per CDC. This opens up access under the FDA Emergency Use Authorization (EUA) that regulates public health and clinical laboratories.
COVID-19 testing is recommended for:
- Those most likely to have been infected (close contacts of confirmed cases).
- Those most at risk of complications from infection (those over 60 and those with chronic medical conditions that increase risk).
- Those who work in critical occupations or who could transmit to those most at risk of complications (healthcare and public safety workers).
COVID-19 testing is prioritized by organizations for various reasons, such as limited availability of testing or by public health agency priorities.
Labs & Testing:
Testing capacity remains limited in our region. Although the availability of commercial laboratory testing has increased, some labs have more capacity to test than others for various reasons.
Ability of clinicians to test requires all of the following:
- Availability of a lab.
- Availability of specimen collection materials (swab and viral transport media).
- Availability of appropriate PPE to collect and package specimens safely.
None of these are sufficient in our community at this time.
Recent changes:
- Do not send patients to the Whatcom County Health Department with an Rx for testing or ask them to request testing from us. If you are unable to test a patient, please call WCHD to discuss options or referring, to see if the testing meets our priorities, and to schedule specimen collection if approved. We do not collect specimens on a drop-in basis.
- Washington State Public Health Laboratories (WA- PHL) will accept specimens meeting specific criteria directly from clinicians (3/16/20 update link below). Specimens from the following patients can be sent to WA-PHL:
- Healthcare workers
- Patients in other public safety occupations (e.g., law enforcement, fire fighter, EMS)
- Patients involved in an illness cluster in a facility or group (e.g., healthcare, school, corrections, business)
- Patients with no health insurance
- Clinicians can directly submit specimens for testing for patients meeting the above criteria. Local public health approval is not required, nor is preapproval required by DOH.
- WCHD is available to consult if there are questions about whether to submit specimens to DOH or to use a commercial lab. https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/Interim-2019NovelCoronavirusQuicksheetProviders.pdf
- New WA DOH Return to work Guidance for healthcare workers (HCWs) and first responders who have confirmed COVID-19 infection or or are asymptomatic with High or Medium Risk Exposures* to a known case of COVID-19
Changes to Specimen Selection
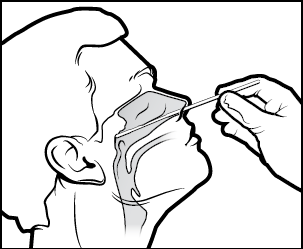
CDC issued new guidance on specimen collection (3/13/20) recommending collecting and testing a single NP swab:
“For initial diagnostic testing for COVID-19, CDC recommends collecting and testing an upper respiratory nasopharyngeal swab (NP). Collection of oropharyngeal swabs (OP) is a lower priority and if collected should be combined in the same tube as the NP. Collection of sputum should only be done for those patients with productive coughs. Induction of sputum is not recommended. Specimens should be collected as soon as possible once a PUI is identified, regardless of the time of symptom onset. Maintain proper infection control when collecting specimens.”
Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
Changes to Infection Control Guidance
It is important to conserve PPE for recommended uses.
- Respirators (N-95, CAPR, PAPR) should be prioritized for use with aerosol-generating procedures. Nasopharyngeal swab collection is considered aerosol-generating.
- Guidelines are provided for prioritizing use of available PPE when supplies are limited.
- Healthcare Supply of Personal Protective Equipment https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe-index.html
- Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings (updated 3/10/20)
- https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html
Patient Instructions for Isolation & Quarantine
If COVID-19 is being considered, whether testing is performed or not, please provide patients with the following guidance documents so that they know how to best prevent the spread of infection to other people, and to minimize phone calls to your office and to public health.
- Patients with confirmed or suspected COVID-19 https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/COVIDcasepositive.pdf
- Patients who were exposed to a confirmed COVID-19 case https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/COVIDexposed.pdf
